Exploring the Crucial Role of H2A.Z.1 in Memory and Gene Expression
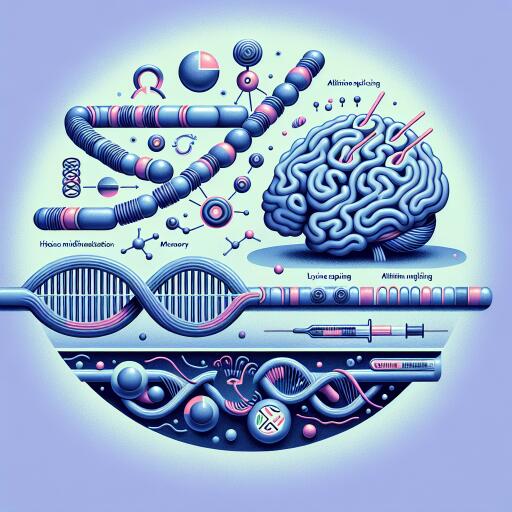
In a groundbreaking study, researchers have delved deep into the intricacies of the histone variant H2A.Z.1, uncovering its pivotal role in memory formation, transcription, and alternative splicing, all regulated by specific lysine modifications. Histones, the protein components of chromatin, play a significant role in gene regulation. Among them, H2A.Z.1, a variant of the histone H2A.Z, has emerged as a critical player in the neural landscape, particularly in the context of memory and gene expression.
At the heart of this study is the exploration of the acetylation patterns of H2A.Z.1, specifically at lysines 7, 9, and 11. Acetylation, a form of chemical modification where an acetyl group is added to a molecule, can significantly impact the functionality of proteins, including histones. To dissect the role of these particular lysines in memory processes, researchers employed a strategic mutation approach, substituting these lysines with glutamine (to mimic acetylation) or alanine (to prevent acetylation), offering a nuanced understanding of the acetylation effect.
The study utilized genetically modified mice, enabling the targeted deletion of H2A.Z in the hippocampus, a brain region integral to memory formation, to examine the resulting effects on memory and gene expression. This manipulation was achieved through advanced genetic engineering techniques, including AAV-mediated gene delivery, to precisely modify H2A.Z.1 expression and incorporate mutations that mimic or block acetylation.
Following meticulous behavioural assessments, including object location memory (OLM) and contextual fear conditioning, the study not only confirmed the significance of H2A.Z.1 in memory formation but also illuminated how its acetylation state influences memory strength. Remarkably, the mutation experiments provided compelling evidence that mimicking acetylation at key lysine residues enhances memory, whereas blocking acetylation has the opposite effect, underscoring the regulatory role of H2A.Z.1 acetylation in neural processing.
Beyond behaviour, the study extended into the realm of gene expression and alternative splicing. Using high-throughput sequencing technologies, the researchers were able to profile the transcriptional landscape and splicing patterns within the hippocampus, revealing that the manipulation of H2A.Z.1 not only affects memory capacity but also induces widespread changes in gene expression and alternative splicing, potentially linking these molecular changes to observed behavioural outcomes.
The findings further emphasized the complexity of histone regulation, where H2A.Z.1’s lysine acetylation mediates memory function through a delicate balance of transcriptional regulation and alternative splicing. Intriguingly, the study’s gene ontology pathway analyses pointed to specific biological processes and pathways that are impacted by H2A.Z.1 modulation, providing insights into the molecular underpinnings of memory and the broader implications of histone modifications.
In conclusion, this research marks a significant step forward in our understanding of the epigenetic mechanisms underpinning memory formation and gene expression. By shedding light on the functional role of H2A.Z.1 acetylation, the study not only expands our knowledge of histone biology but also opens new avenues for therapeutic interventions targeting epigenetic regulators in neuropsychiatric disorders. As we continue to unravel the complexities of the histone code, the hope is that these insights will eventually translate into novel strategies for enhancing memory and combating cognitive decline.
The study, a testament to the power of modern biotechnology and neuroscience, paves the way for future research into histone variants and their myriad roles in brain function and beyond, heralding a new era of molecular neuropsychopharmacology.